Why Diversity in Clinical Trials Matters for Cystic Fibrosis Research
Post Preview
Key Takeaways:
- Diversity in clinical trials ensures research findings are robust and applicable across diverse populations, enriching scientific insights and treatment relevance.
- Understanding genetic variations across different communities can lead to the development of more effective, tailored treatments for Cystic Fibrosis.
- Increasing diversity in clinical trials can significantly enhance equitable healthcare outcomes, ensuring no demographic is marginalized in medical advancements.
Introduction
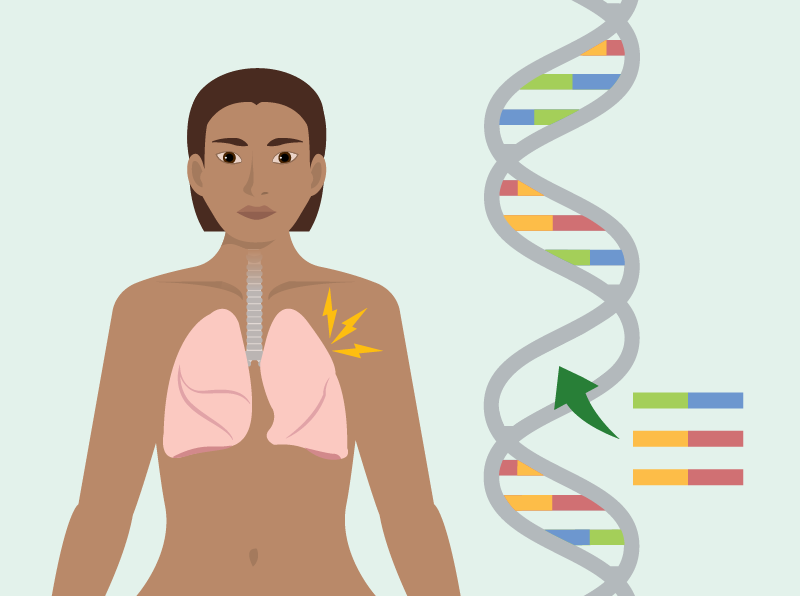
Cystic Fibrosis (CF) is a complex, life-shortening genetic disorder affecting over 70,000 individuals worldwide, particularly impacting the lungs and digestive systems. While significant advancements have been made in medical treatments and management strategies for CF, a substantial gap remains in ensuring that clinical trials reflect the diversity of populations most affected by the disease. By prioritizing diversity in cystic fibrosis drug trials, researchers can enhance the effectiveness and universality of treatments, tailoring them to meet the genetic and environmental intricacies found across various ethnic and racial groups.
The push for diversity in clinical trials is not only an ethical imperative but a scientific necessity. Clinical trials that include a wide range of demographics facilitate a more nuanced understanding of the disease’s manifestation and treatment responses in varied populations. This article delves into the critical significance of diversity in CF clinical trials and its profound implications for achieving equitable and effective healthcare outcomes on a global scale.
The Importance of Representation in Research
Why Diversity Matters
The genetic mutations that cause CF exhibit significant variation across different racial and ethnic groups worldwide. Historically, CF research has centered predominantly on populations of European descent, where the prevalence of the disease is higher. This narrow focus risks ignoring unique genetic markers and socio-environmental factors that can significantly influence the efficacy and safety of treatments in other populations. Without inclusive representation, clinical trial findings risk being overly generalized, potentially overlooking essential nuances necessary for effective treatment among underrepresented groups.
Incorporating diverse demographics into clinical research acknowledges and values these differences, enabling the development of treatment regimens that are both inclusive and precise. The National Institutes of Health (NIH) emphasize the importance of this principle, advocating for inclusive research practices to ensure that medical discoveries are beneficial and applicable across a broad spectrum of communities, particularly those historically underrepresented in clinical studies. Addressing this gap through diverse participation in trials can lead to medical advancements that serve the global CF community more effectively by providing solutions considering the complex interplay of genetic and environmental factors.
Barriers to Diversity in Clinical Trials
Challenges to Representation
Numerous barriers exist to achieving diversity in clinical trials, with socio-economic challenges among the most significant. These challenges often restrict access to healthcare and research participation opportunities, particularly among minority communities. High transportation costs, inflexible work schedules, and a lack of access to information about ongoing trials further exacerbate these barriers.
Strategies for Improvement
To overcome these hurdles, a comprehensive, community-focused approach is essential. Building partnerships with local community organizations is crucial for fostering trust and disseminating vital information about the purpose and benefits of clinical trials. Additionally, developing recruitment materials that are culturally sensitive and available in multiple languages can significantly enhance accessibility for non-native English speakers. Providing logistical support—such as transportation and flexible scheduling options—also makes participating in trials more feasible and attractive, increasing diversity and representation in clinical research across diverse societal sectors.
Impact on Cystic Fibrosis Research and Treatment
Genetic Insights and Treatment Effectiveness
Diverse clinical trials present unparalleled opportunities to explore the genetic complexities of CF, facilitating the identification of unique markers that may vary among different populations. This understanding is invaluable for advancing personalized medicine approaches, which aim to tailor interventions based on individual genetic profiles, thereby enhancing treatment efficacy. Personalized therapies significantly improve care quality while reducing the likelihood of adverse drug reactions, which can occur when treatments do not consider population-specific genetic differences.
Organizations like the Cystic Fibrosis Trust advocate for inclusive research to uncover treatment pathways reflecting human genetic diversity. Their efforts underscore the necessity for comprehensive data collection to ensure that healthcare strategies effectively address the diverse needs of CF patients, ultimately enhancing treatment outcomes across various demographics. As our understanding of CF’s genetic landscape deepens, the potential to develop more effective and universally applicable treatments expands.
Steps Toward Equitable Healthcare
Creating a healthcare system that equitably serves a globally diverse population mandates a strong commitment to inclusivity in research. This commitment guides healthcare practices toward ensuring balanced representation and equity in policy-making. Diverse clinical trials ensure that newly developed treatment protocols are universally applicable, reducing health disparities that historically marginalized certain groups. Such practices are essential for enhancing the inclusivity of healthcare and ensuring fairness, so that advancements are accessible to all segments of society.
By prioritizing inclusivity, the healthcare community can address structural disparities, leading to the development of treatments effective across all demographic groups. This requires collaboration among scientists, healthcare providers, policymakers, and communities to dismantle systemic inequalities and establish new standards for comprehensive healthcare access that will benefit a global society. Inclusivity in research offers the promise of medical breakthroughs that cater to diverse genetic backgrounds, supporting a healthier future for everyone.
Conclusion
Diversity in clinical trials is more than a commitment to equity—it is central to fostering an inclusive, effective healthcare system equipped to meet future challenges. For Cystic Fibrosis research, embracing diversity democratizes access to medical advancements, ensuring these innovations are relevant and effective for all populations. Ongoing efforts in cystic fibrosis drug trials offer a path forward for achieving health equity in medical advancements, paving the way for significant strides towards eliminating longstanding disparities that have hindered fair access to effective treatment and healthcare quality.